Catalyst Development
Background
The anode and cathode electrocatalysts are crucial components of the membrane electrode assembly (MEA) of the PEMWE, as they promote the half-reactions, namely oxygen and hydrogen evolution reaction (OER and HER). Currently, noble metals are used in PEMWE catalysts, such as platinum (Pt)-based materials and iridium/ruthenium (Ir/Ru)-based compounds. However, these metals pose challenges for the large-scale commercialization of PEMWE, because their availability is affected by their low natural abundance, leading to high costs. These two factors directly impact the sustainability of
the technology in the long term.
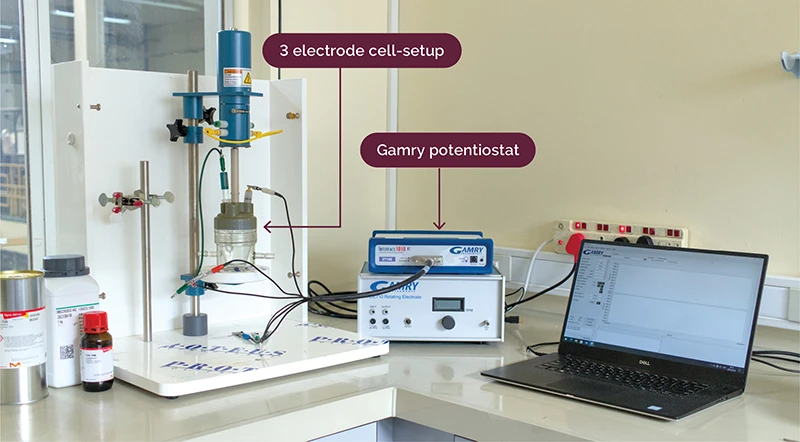
Research into non-platinum group metal (non-PGM) materials for catalyst development in PEMWE systems is currently being pursued by H2GEN. This work is built upon the significant advances made in the design and synthesis of high-performance transition metal(TM)-based catalysts, including TM-based alloys and TM-based compounds (TMXs, where X = Ni, Co, Mo, etc.), which are investigated to enable commercial large-scale water electrolysis without dependency on scarce precious metals. Ex-situ characterization is conducted using a potentiostat and three-electrode-cell setup to simulate the HER or OER half-cell reactions.
In this manner, key performance parameters such as activity, stability, and reaction kinetics are measured. Materials that show promise in these tests are then advanced to in-situ characterization in a single-cell PEMWE, where their performance under real-world conditions is evaluated, allowing an assessment of how these catalysts function when integrated with other components in the electrolysis cell.
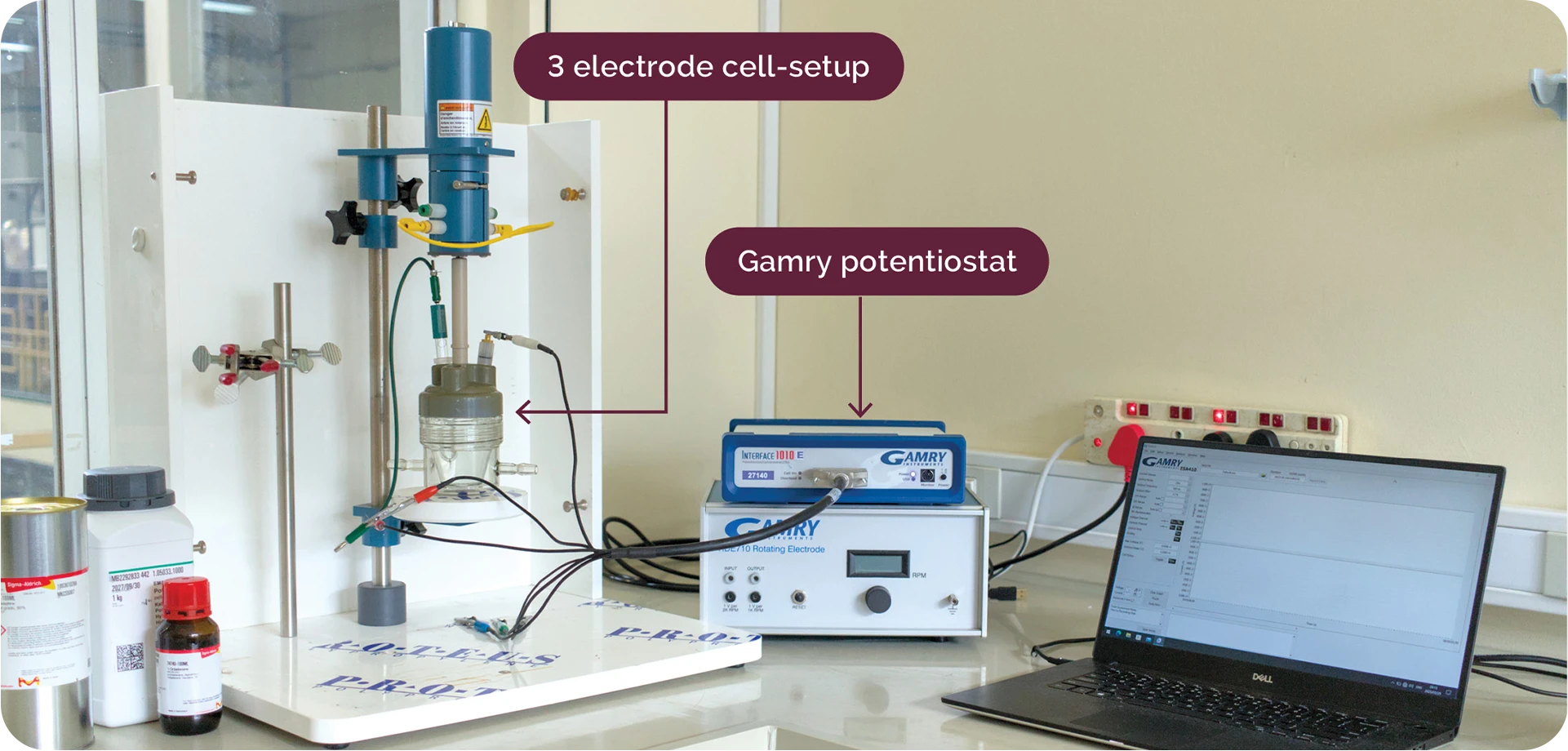
Ex-situ 3-electrode cell setup for catalyst electrochemical characterization