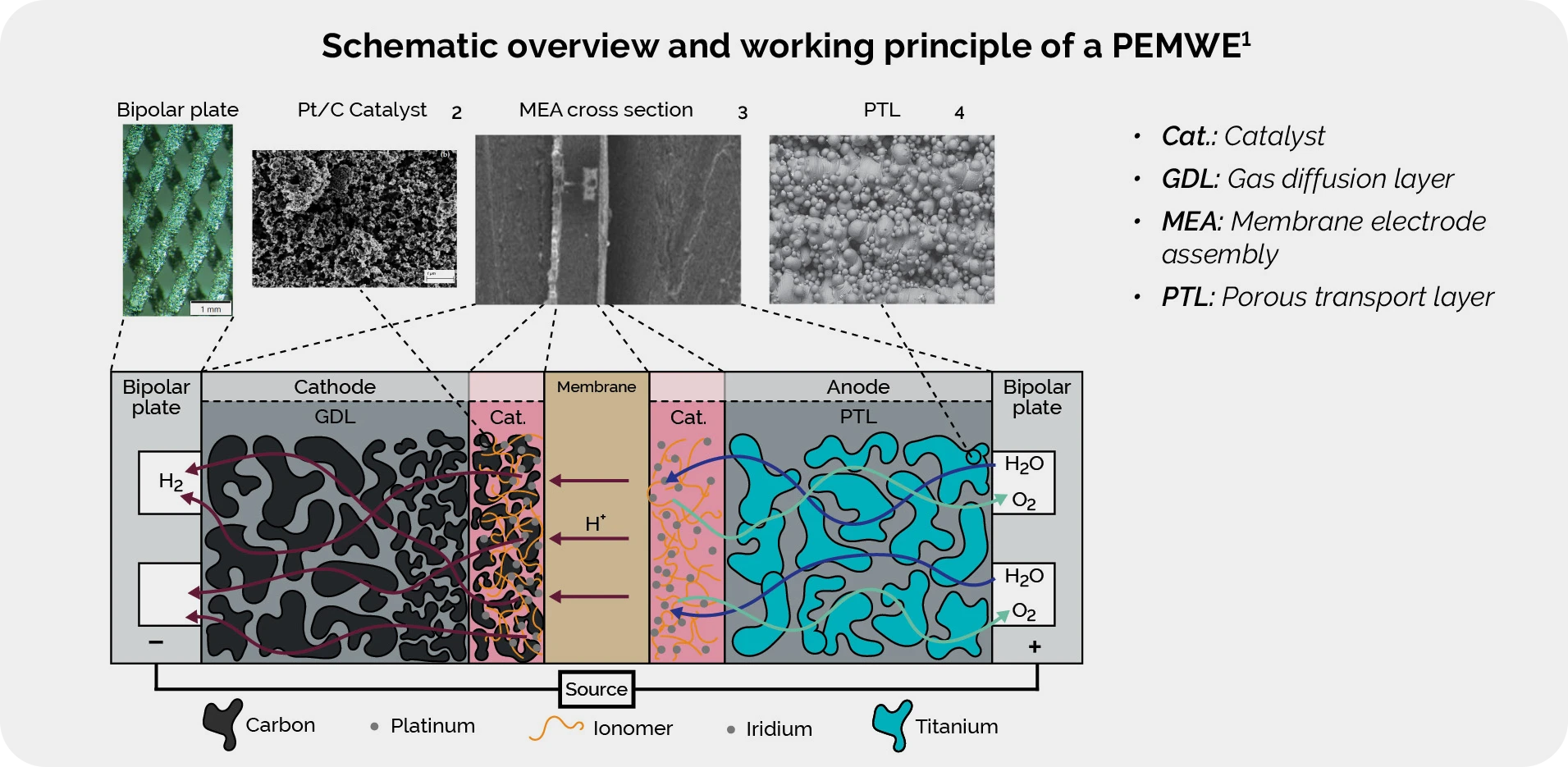
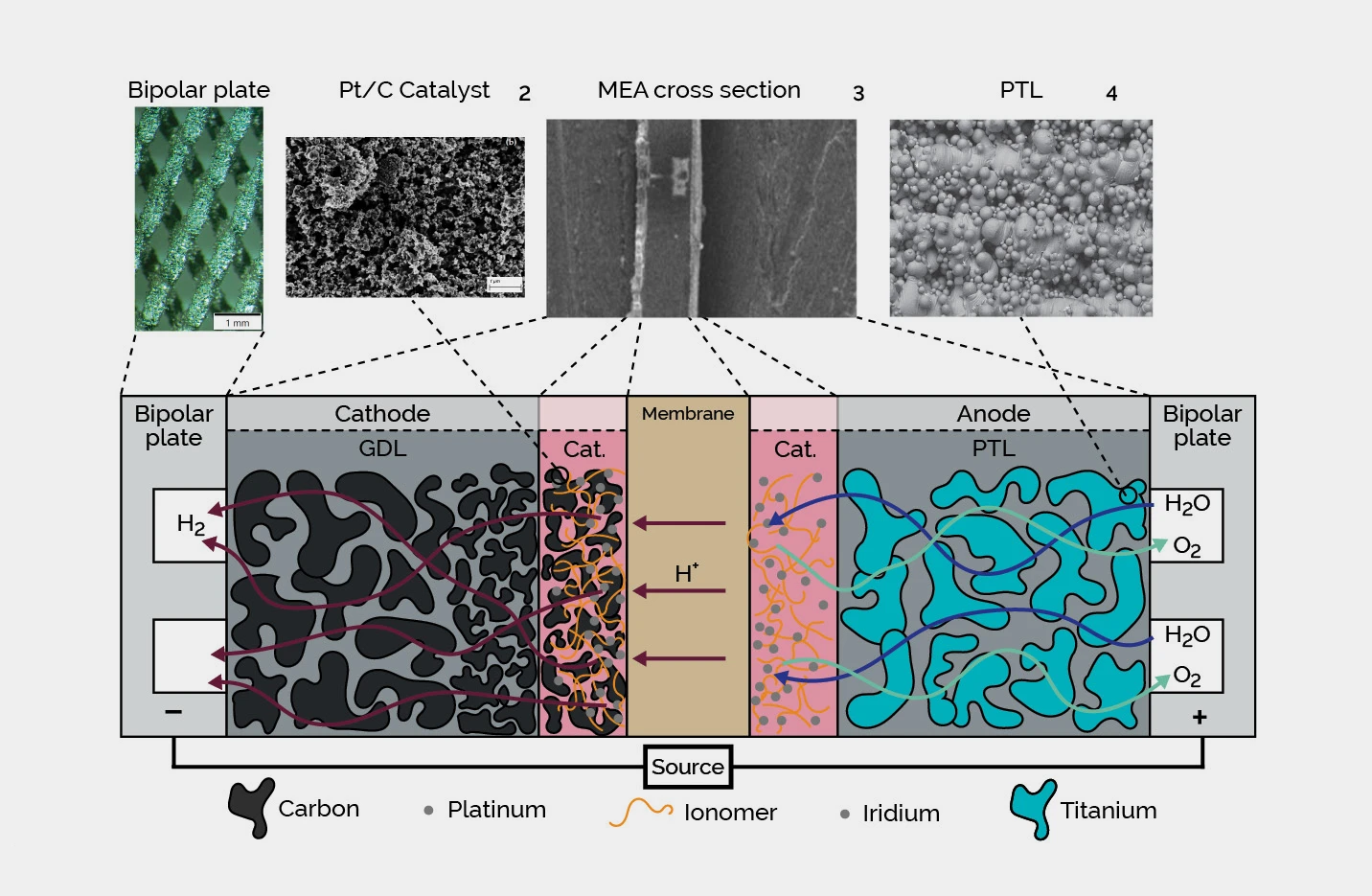
| E. Mukoni, K.S. Garner, "Multi-Objective Non-Dominated Sorting Genetic Algorithm Optimization for Optimal Hybrid (Wind and Grid)-Hydrogen Energy System Modelling," MDPI: Energies, Volume 15(19), 7079, ISSN 1996-1073, 2022. |
Systems and Integration |
Optimisation studies |
Journal |
2022 |
- |
| C.E. Bosman, R.W. McClelland Pott, S.M. Bradshaw, "Design, modelling and simulation of a thermosiphon photobioreactor for photofermentative hydrogen production," Biochemical Engineering Journal, Volume 186, 108582, 2022. |
Alternative Production |
Microbial Photobioreactors |
Journal |
2022 |
Microbial Photobioreactors |
| J.H.H. Giliomee, T. Zenner, M.J. Booysen, "Swappable green hydrogen trailers as an additional energy source to electric minibus taxis," African Transport Studies, Volume 1, 100001, 2023. |
Storage and Usage |
Fuel cells |
Journal |
2023 |
- |
| C.E. Bosman, R.W. McClelland Pott, S.M. Bradshaw, "Modelling and testing of a light reflector system for the enhancement of biohydrogen production in a thermosiphon photobioreactor," Journal of Biotechnology, Volume 361, Pages 57-65, 2023. |
Alternative Production |
Microbial Photobioreactors |
Journal |
2023 |
Microbial Photobioreactors |
| C.E. Bosman, P. Van Wyk, R.W. McClelland Pott, S.M. Bradshaw, "The effect of diurnal light cycles on biohydrogen production in a thermosiphon photobioreactor," AMB Express, Volume 13, 2023. |
Alternative Production |
Microbial Photobioreactors |
Journal |
2023 |
Microbial Photobioreactors |
| E. Mukoni, K.S. Garner, C. Van Staden, "Hybrid system optimization model for hydrogen production, case study: South Africa," International Conference on Environment and Electrical Engineering (EEEIC), 2023. |
Systems and Integration |
Optimisation studies |
Conference |
2023 |
- |
| C.E. Bosman, R.W. McClelland Pott, S.M. Bradshaw, "The effect of light emission spectrum on biohydrogen production by Rhodopseudomonas palustris," Bioprocess and Biosystems Engineering, Volume 46, pages 913–919, 2023. |
Alternative Production |
Microbial Photobioreactors |
Journal |
2023 |
Microbial Photobioreactors |
| C.J. Abraham, T. Zenner, M.J. Booysen, A.J. Rix, "Decarbonising South Africa’s paratransit with hydrogen: a simulated case study," Conference Proceedings (Electrical and Electronic Engineering), 2023. |
Systems and Integration |
Techno-economics |
Conference |
2023 |
- |
| J.A. Woods, A.J. Rix, C.Y. Van Staden, "A review of electrolyser modelling for hydrogen production coupled with renewable energy sources," South African Sustainable Energy Conference (SASEC), 2023. |
Electrolysis |
Electrolyzer modelling |
Conference |
2023 |
Electrochemical Water Electrolysis |
| A.M.M. Petersen, J.F.F. Gorgens, "Flowsheet analysis of bio-derived hydrogen as a surplus product at sugar mills and associated biorefineries from processing residues," International Journal of Hydrogen Energy, Volume 49, Part A, Pages 225-237, 2024. |
Storage and Usage |
Hydrogen Integration in Biorefineries |
Journal |
2024 |
Hydrogen Integration in Biorefineries |
| A. Dell Orto, C. Trois, "Double-Stage Anaerobic Digestion for Biohydrogen Production: A Strategy for Organic Waste Diversion and Emission Reduction in a South African Municipality," MDPI - Sustainability , Volume 16(16), 7200, 2024. |
Alternative Production |
Biohydrogen production |
Journal |
2024 |
- |
| E. Grobler, G.M. Teke, B.A. Cho, D. Zhang, R.W.M. Pott, "Synergistic interplay of photobioreactor mixing and static mixer configuration on Rhodoseupdomonas palustris's growth and biohydrogen production," Fuel, Volume 378, 132861, 2024. |
Alternative Production |
Microbial Photobioreactors |
Journal |
2024 |
Microbial Photobioreactors |
| G.M. Teke, B.A. Cho, C.E. Bosman, Z. Mapholi, D. Zhang, R.W.M. Pott, "Towards industrial biological hydrogen production: a review," World Journal of Microbiology and Biotechnology, Volume 40(37),2023. |
Alternative Production |
Biohydrogen production |
Journal |
2023 |
- |
| A. Dominic, J.H. Giliomee, T. Zenner, M. Winter, G. Schullerus, M.J. Booysen, "Green Hydrogen for Future Energy Demand in Germany," IEEE International Energy Conference and Exhibition (EnergyCon), 2024. |
Systems and Integration |
Techno-economics |
Conference |
2024 |
- |
| G. Ter Haar, C. McGregor, "Additive manufacturing of titanium porous transport layers for enhanced performance in proton exchange membrane water electrolysis," 2024 RAPDASA-RobMech-PRASA-AMI Conference: Unlocking Advanced Manufacturing, Volume 406, 2024. |
Electrolysis |
Additive Manufacturing of Cell Components |
Conference |
2024 |
Additive Manufacturing of Cell Components |
| R. Laubscher, P.G.G. Rousseau, E. De Villiers, "Thermofluid process simulation of wet biomass and ammonia co-firing in an industrial watertube boiler," Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy, Volume 239(2), 2024. |
Storage and Usage |
Combustion of Hydrogen–Hydrocarbon fuel mixtures |
Journal |
2024 |
Combustion of Hydrogen–Hydrocarbon fuel mixtures |
| G. Ruzive, K.S. Garner, "Optimising Hybrid Energy Solutions for Hydrogen Production in South Africa," 4th International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), 2024. |
Systems and Integration |
Optimisation studies |
Conference |
2024 |
- |
| R. Béres, N. Mararakanye, C. Auret, B. Bekker, M. van den Broek, "Analysing the prospects of grid-connected green hydrogen production in predominantly fossil-based countries – A case study of South Africa," International Journal of Hydrogen Energy, Volume 83, Pages 975-986, 2024. |
Systems and Integration |
Hydrogen strategy and roadmaps |
Journal |
2024 |
- |
| G.H. Creasey, A.W. Moelich, J.W. Rodriguez Acosta, T.P. Shalvey, D.A. Garcia-Osorio, T.W. McCallum, L. O Neil, J. Zhu, S. Halder, R. Ai, J. Major, A.J. Cowan, C. McGregor, A.G. Kafizas, A. Hankin, "Up-Scaling Solar Hydrogen Production: Development and Demonstration of Photoelectrochemical Reactors," ECS Meeting Abstracts, 2024. |
Alternative Production |
Photoelectrochemical Water Electrolysis |
Conference |
2024 |
Photoelectrochemical Water Electrolysis |
| C. Mcgregor, B.D. Young, D. Hildebrandt, "Risk assessment framework for green hydrogen megaprojects: Balancing climate goals with project viability," Applied Thermal Engineering, Volume 262,125197, 2025. |
Systems and Integration |
Techno-economics |
Journal |
2025 |
- |
| Agora Industry, Fraunhofer IEE, Stellenbosch University, "Power-to-X Allocation Study for South Africa,", 2025. |
Systems and Integration |
Power-to-X Systems Integration |
Report |
2025 |
Power-to-X Systems Integration |
| A. Hankin, G. Creasey, A. Moelich, J.R. Acosta, J. Videira, J. Zhu, S. Halder, C. McGregor, A. Kafizas, "Integrating concentrated solar power with a prototype photoelectrochemical reactor," Preprint, 2025. |
Alternative Production |
Photoelectrochemical Water Electrolysis |
Journal |
2025 |
Photoelectrochemical Water Electrolysis |
| G. Ter Haar, C. McGregor, "Additive manufacturing of titanium porous transport layers for efficient PEM water electrolysis," Materials Today Sustainability, Volume 32, 101219, 2025. |
Electrolysis |
Additive Manufacturing of Cell Components |
Journal |
2025 |
Additive Manufacturing of Cell Components |
| C. Fenner, J. Van Der Spuy, R. Laubscher, "Predicting combustor performance for hydrogen-propane fuel blends in gas turbines: A coupled thermofluid and chemical reactor network model," International Journal of Hydrogen Energy, Volume 195, 152511, 2025. |
Storage and Usage |
Combustion of Hydrogen–Hydrocarbon fuel mixtures |
Journal |
2025 |
Combustion of Hydrogen–Hydrocarbon fuel mixtures |
| P. Thiele, Y. Yang, Y. Liu, M. Wick, S. Pischinger, "Realistic accelerated stress tests for PEM fuel cells: Validation of load profile optimization via lifetime prognosis in fuel cell electric vehicles," International Journal of Hydrogen Energy, Volume 203, 152594, 2026. |
Storage and Usage |
Fuel cells |
Journal |
2026 |
- |